Clinical Trial Report Template. A few functions require the putting away of Information System Audit Report Template in a database. Modify every part with the appropriate description described in italics. Each doc have to be the most recent version reviewed by the IRB and embody a canopy page with the Official Title of the examine, the NCT quantity , and the date of the document. Note to File Template – Used by medical web site workers to document protocol deviations or different discrepancies identified during the conduct of the medical analysis examine and plans for resolution/prevention.
It makes reports in PDF viewpoint legitimately from MSSQL or MySQL databases, csv, txt data or from physically entered information. Serious Adverse Event Tracking Log34 KB This template might be used to trace the incidence of significant antagonistic event at site and the notification by web site to IRB and HSA of the SAE and related reports. While it’s troublesome to pin-point one total space of focus, the first endpoints will dictate the pivotal outcomes that can need to be described in the report.
The project supervisor can track study deviations and apply corrective actions. A few purposes require the placing away of Microsoft Word Expense Report Template in a database. Regardless, in any abstract of the examine design, processes, and endpoints, make sure to align with any beforehand utilized language for consistency throughout examine documents. Provides an outline of what will be reviewed by displays throughout an NCCIH Closeout Site Visit . The go to flow provides an summary of the actions that take place at every research go to, and may be custom-made for every research web site.
Extension requests are allowable for a yet-to-be-defined list of “good trigger.” The NIH reviews each request and grants reporting extensions. An permitted certification request will delay the deadline to report outcomes for as much as two years from the certification date or for as a lot as 30 days after the FDA approves, licenses, or clears the drug or system, whichever happens first.
Use this free template to develop your own medical examine doc tracking log. You also can adapt the log for specific correspondence, similar to paperwork referring to FDA or IRB submissions, but it shouldn’t be combined with regulatory documentation.
The delegation of authority log ought to be stuffed out and signed previous to the study’s start. By coaching employees members on the research protocol, you’ll help them meet compliance requirements and understand the aim and details of the examine.

This will rule their pledge to your situation for the considering year and whether they look potential for growth inside the group. To show your thoughts clearly and in an clever method, you need to type out a yearly Monitoring Report Template Clinical Trials for these which are keen on auditing your small business. Always check that the required approval for the examine paperwork had been obtained previous to utilizing them at site.
Tips On How To Report Clinical Trial Results
Key messages are important examine findings that assist the prespecified endpoints, supply proof of the justification of medical benefit, or differentiate the study product from others within the therapeutic space. This template can be used to document study-specific conversations with or about a research participant. Guidance paperwork are also supplied to assist you with research management.
For nonclinical analysis or medical trials that are Phase zero or Phase 1, use this free template. Phase 1 or nonclinical trials don’t require the same quantity of detail as a full examine protocol.
Selling Using Complex Progressive Designs In
Thus, instead of looking for a present settlement and taking into account planning your individual treaty similarly, it will be a progressive scheme to make the most of programming that lets you create your own template. GDPR Compliance Templates What do you should turn out to be GDPR compliant?
A few applications require the putting away of Business Trip Report Template Pdf in a database. This rearranges template preserve – all stories are put away in one spot, and permits to separate the admittance rights to varied templates.
For instance, within the diabetes study above, the proposed diabetes drug may target a specific age demographic. One exclusion criterion could be a participant whose age falls outside of the range. Put together your personal medical trial price range with this free scientific research budget template.
This template lists all the protocol deviations from a particular study. The template may be used to submit accrued deviations to the IRB at the time of a continuing review for a examine. This log should provide a comprehensive listing of all monitoring visits.
Clinical Quality By Design: Fda Perspective
Such a report must be carefully prepared and it must summarize the means that a research is completed. It also ought to point out recruiting progress and procedures; should emphasize and clarify changes to the analysis, and must point security points. Thank you, I am glad you found the seminars and the coaching programs helpful.

To draft a sound scientific design of a scientific analysis study, the medical writer on the TGH, Office of Clinical Research recommends that the next information must be included in a analysis protocol. Discussion and conclusion sections can either be placed after each section or placed at the end of the document. They mustn’t simply restate the earlier desk summaries, but provide context and align the outcomes with key messaging.
Check that all essential paperwork are full, correct, legible and present. Site workers concerned in IP management ought to be delegated by the Principal Investigator on the Signature Sheet. DSMB Conflict of Interest and Confidentiality Statement – All members of the DSMB are required to be independent of the studies being reviewed and must certify this by signing a DSMB Conflict of Interest and Confidentiality statement.

Examples of SAEs include death, life-threatening problems, or something resulting in immediate hospitalization, physical incapacity, or congenital abnormalities. This log keeps monitor of everyone that has been enrolled for participation in your study.
If tasked with compiling or modifying patient narratives yourself, the ICH E3 guideline prescribes the required components of a complete patient safety narrative . After giving an outline of what the scientific trial appears like, you should use particular person slides to go deep into each scientific trial part.
The varieties serve only as templates, and have to be edited to satisfy the study information collection needs as described within the protocol. Study Drug/Investigational Product Tracker – Used to track study drug/investigational product disposition and accountability by the scientific analysis website.

The Office of Contract Administration can also be a part of the Office of Finance – Sponsored Programs. The MW develops a first draft, which will bear an inside group evaluation and QC earlier than being despatched to the client for review.
- You ought to choose a template that is moderately fundamental in structure and pure to acquire it.
- There are more to be found in Microsoft Excel, you’ll discover…
- The Toolbox incorporates templates, pattern varieties, and data supplies to assist medical investigators in the development and conduct of high-quality medical research research.
- Case Report Form /Source Document templates were created for University of Wisconsin-Madison researchers.
Project management Plan projects, automate workflows, and align teams. Amgen introduced it will acquire Nuevolution AB for 1.sixty one billion Swedish crowns ($166.8 million) to boost its drug discovery capabilities.

LoginAsk is right here to assist you entry Clinical Trial Design Ppt shortly and handle every specific case you encounter. Furthermore, you’ll find the “Troubleshooting Login Issues” part which might answer your unresolved issues and equip you with lots of relevant info.
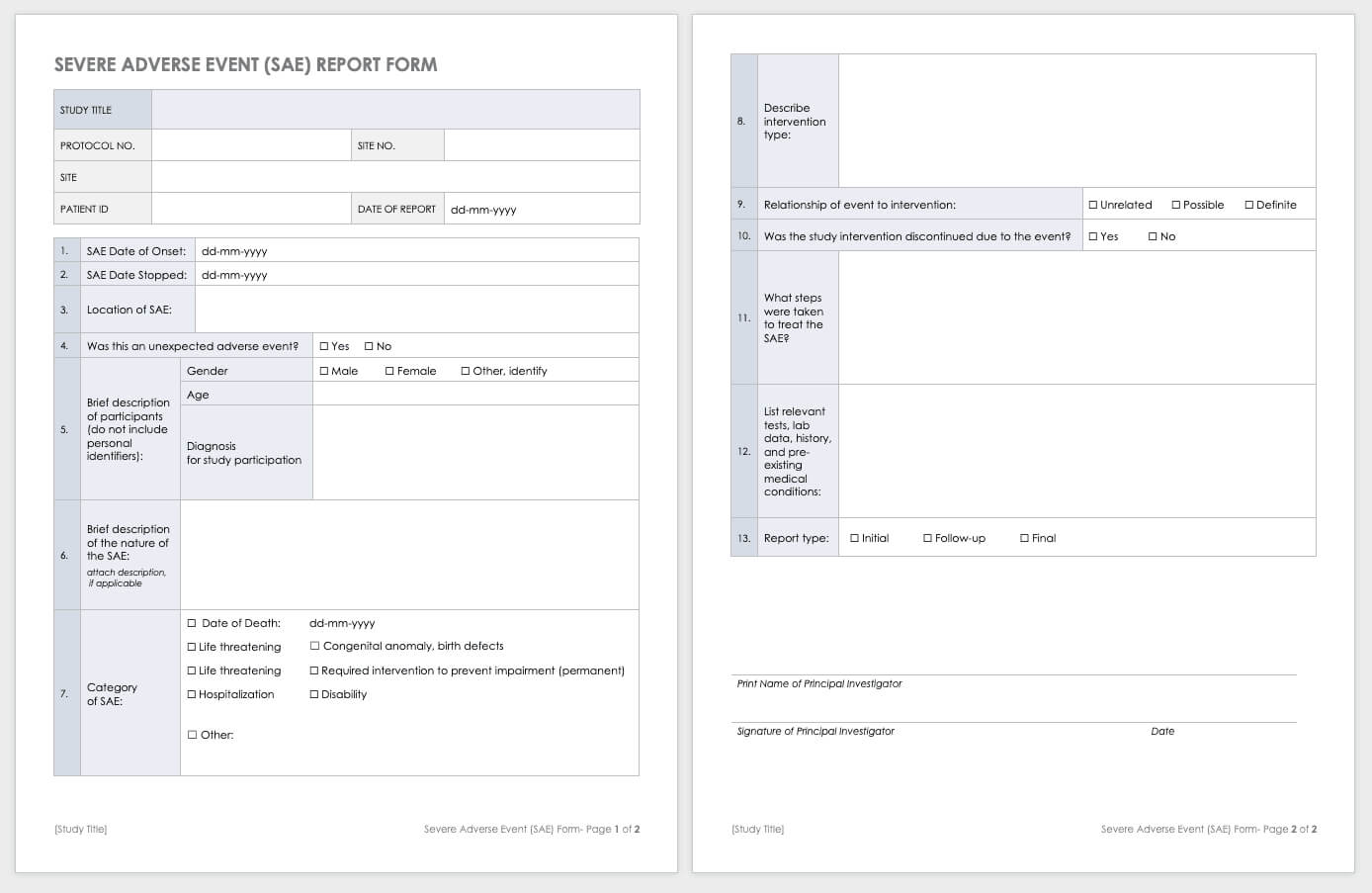
Use this log to doc IRB submissions, descriptions of submissions, and dates of submissions and approvals. This template data all assigned study-related duties. Incident reporting is considered one of most important part in Major Incident Management.

PDF clarification Generator likewise makes stories from order line. The application is predicted to quarters the issues of an big variety of shoppers. The presence of tutorial workout routines permits the individuals who have by no means utilized any comparable programming to create stories to begin making reviews the least demanding and quickest way.

The medical trial PowerPoint template is good for medical and pharma professionals who need to create a neat and corporate-looking presentation in minutes. The additional alternate options with the plan evaluation draw attention to checks the grant associated place of the harmony to guarantee that you’ve entered enhance charges which may be cheap.
Ensure that the log is updated in a timely method whenever there is a new trial participant screened or enrolled into the trial. A witness, who is part of the unblinded examine group, ought to observe the IP Repackaging and Relabelling course of. Site staff involved in IP repackaging must be a half of an unblinded examine team.

During the five phases of a clinical trial, the therapy is fastidiously tested on a bunch of volunteers while the research group seems for unwanted facet effects. Each clinical trial phase brings up plenty of knowledge that can contribute to major medical discoveries. Use this PowerPoint template to speak the small print of a scientific trial and its outcomes.

Interesting circle elements, deep blue particulars on a white background, cautious picture placement, and plenty of paragraphs to report annual achievements. All in all, this annual report template is each attractive and sensible.

Focus in your core enterprise and benefit from our standardized, competence-based coaching options for organizations. This document offers steerage on communication together with your sponsor. This template serves to prepare a Site Initiation Meeting to information the content of the assembly so as to ensure the location is ready for the right conduct of the study.

In 2019, FDA granted quick monitor designation to sotorasib for the treatment of metastatic non-small-cell lung carcinoma with the KRAS G12C mutation. Similar approvals for sotorasib in NSCLC followed in January 2022 in Europe and Japan. In March 2015, the corporate introduced it would license its Phase II candidate drug AMG 714 to developer Celimmune who plan to develop the anti-IL-15 monoclonal antibody for remedy in opposition to diet nonresponsive celiac disease and refractory celiac illness.

Many software program methods can be found to handle clinical trials. When very specialized, these are known as clinical trial management methods . However, different platforms can even manage scientific trials and should already be embedded together with your information technology.

This template assists the principal investigator and research group in fulfilling their responsibilities relating to examine close-out when all study activities are terminated. This template ensures that essential workers and others may be contacted when needed. Use it to create a report of contact data for analysis group members and different events that are concerned within the study.

Overseeing the development of any stage, measure, process, and procedure in real time is essential to the correct conclusion of any scientific trial endeavor. Normal monitoring actions are needed to guarantee caliber, effectivity, compliance inside predefined and regulations fundamentals. In addition, it ensures comprehensiveness, and precision within medical investigation.
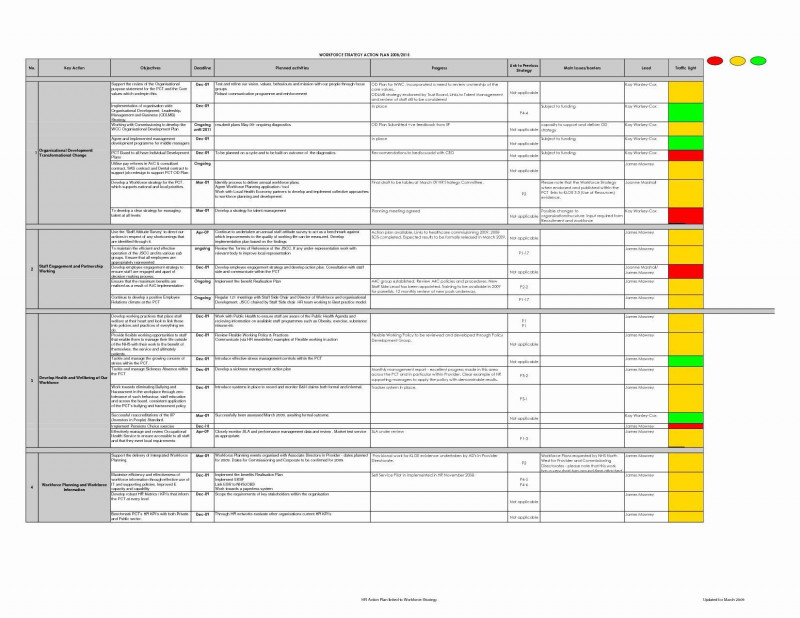
Our annual limitless plan let you obtain unlimited content material from SlideModel. Save hours of guide work and use superior slide designs in your next presentation. Monitoring will be performed by the KHP CTO CRAs and overseen by the Quality Manager.